Written by Jeff Andrews, MD
As the return to in-person is under way, a common question is whether schools are ready to safely welcome back students, teachers, and staff without risking an outbreak of COVID-19. According to the CDC, there is a lower risk of person-to-person transmission of the virus if preventative measures are put in place. These strategies require coordination between schools, state departments of education, and public health divisions to set up programs that ensure ease of use, and regulated practices.
In support of creating this infrastructure, President Biden recently committed to investing more than $10 billion toward building up COVID-19 testing in schools.
In addition to ensuring physical safety, there is a desire to support mental and emotional wellbeing for students. As noted in World Medical & Health Policy, students face significant risks indicates that students face significant risks from the disruption caused by school closures, including a decline in learning, a higher likelihood of dropping out, adverse mental health issues, and other disruptions. From a holistic standpoint, COVID-19 rapid antigen testing goes beyond reassuring students and teachers who test negative and isolating those who test positive: it fast-tracks a safe return to normalcy that is needed in more ways than one.
Rapid testing is one of the five pillars of preventive measures, along with vaccination, wearing a mask, social distancing, and hand washing. In fact, articles and studies in multiple peer-reviewed publications including the New England Journal of Medicine and the British Medical Journal have explained the benefits of serial, rapid antigen testing.
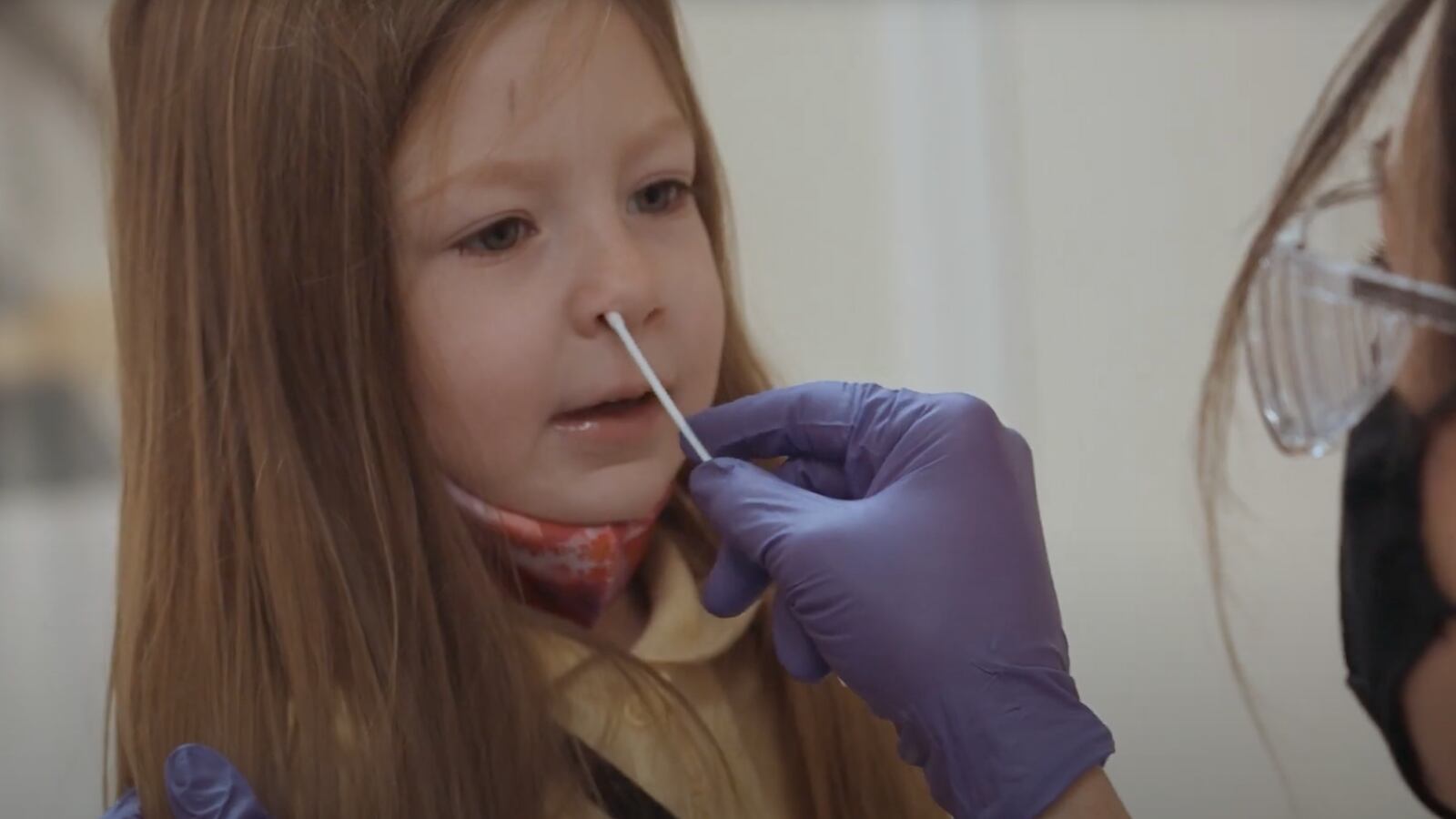
Routine serial testing of students and staff enables those with negative results to resume their normal school routines and will help to identify and isolate positive cases of COVID-19 as early as possible to prevent further spread and facilitate contact tracing and testing. And because antigen tests are fast and simple to use, COVID-19 becomes easy to monitor for even the most novice tester.
One of those testing devices being used in K–12 schools, colleges, and universities across the country is the BD Veritor™ Plus System. A fully portable device, it uses a nasal swab to detect viral proteins and provide results in 15 minutes through a digital readout that eliminates human subjectivity.
Adding to its ease of use, the BD Veritor Plus System can be paired with ImageMover, a companion mobile app that enables more streamlined reporting of testing results. With ImageMover, any school using the BD Veritor Plus System can capture required demographic details of those being tested, upload COVID-19 test results, report results to appropriate stakeholders, and automate reporting to public health agencies. This enables compliance with reporting requirements and significantly reduces manual documentation.
In Delaware, for example, teams from the Delaware Department of Education, the Division of Public Health, and more than 76 charter and private schools across the state collaborated closely to implement testing using the BD Veritor Plus System. Using the BD Veritor Plus System as a major part of its mitigation strategy, these schools have enabled safe in-person learning schools since January.
Screening through serial testing allows schools, school leaders, and administrators to conduct in-person learning with confidence. Continued progress with vaccination, including young people, will also increase public health safety. For those students and teachers with special health concerns, until vaccinated, it will be necessary to continue to provide at-home teaching and learning experiences until the pandemic emergency has ended.
Dr. Jeff Andrews is the Global Medical Affairs Vice President at BD Integrated Diagnostics Solutions. To learn how BD is supporting back-to-school programs on the heels of being granted Emergency Use Authorization (EUA) for its rapid antigen test to be used for SARS-CoV-2 screening through serial testing, visit bdveritor.com.
This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner. For more information, please see bdveritor.com.
Chalkbeat’s editorial staff had no role in writing or preparing this paid content. Learn about our sponsored content policy.